Charcoal
Charcoal is made by charring wood in the absence of air. You can simply make it by filling a pot or a can with pieces of dry wood, closing the pot with a lid and putting it on a moderate fire. Soon you will observe smoke escaping from under the lid. The smoke easily catches fire and burns quietly like gas, with a yellow flame. Turn the pot from time to time so the wood pieces are equally exposed to the heat. As soon as the flame of the woodgas extinguishes, take the pot from the fire and let it cool without opening the lid. This works with any kind of wood.
To prepare a high quality charcoal take a low density wood with a low resin concentration, like willow or hazel wood. Use only well dried young branches, not more than 5 centimeters in diameter and peel off the bark.
Assume that wood (predominantly cellulose plus lignin) can be described chemically as C21H32O14 and charcoal as C7H4O. I'm aware, it's a darn simplification but it facilitates understanding the process. Then the charring process may be described as:
| C21H32O14 | -----> | C7H4O | + | 9 CO | + | 5 CH4 |
+
|
4H2O |
| 'wood' | charcoal | carbon-monoxide | methane | water |
Charring time has little influence on the carbon concentration of the charcoal. Only the charring temperature is (very) important. At 400°C, wood yields 19% charcoal by weight. If charred in a closed container under pressure, then the yield is 60% charcoal, which is why it is generally done that way by the industry.
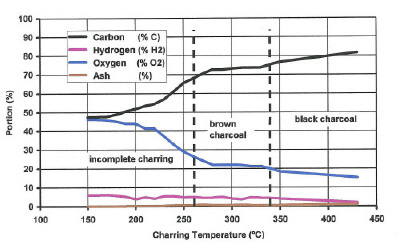
The charring process was investigated first by Violette in 1855 (Zeitschrift für Pyrotechniker, p. 111). He gave the results in a table, since graphs weren't in use then. For easier comprehension I transformed Violette's table to the following graph:.
The Charring of wood, depending on the temperature
 |
||
| Fig 1: The chemical composition of charcoal as a function of temperature | ||
 |
||
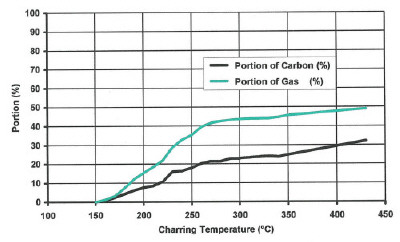
| Fig 2: Production of carbon and woodgas during charring | ||
As demonstrated here by Violette, the charring process starts at 150°C and is very hefty up to 270°. At 400° it is practically finished but continues up to 1,500° with a remaining concentration of 3.8% oxygen and 0.7% hydrogen.
For black powder purposes wood shouldn't be charred at more than 450°. Charred at higher temperatures it looses flammability. Charcoals like those used for barbecuing are "burned to death" and are not suitable for making black powder.
A charcoal charred between 260 and 390°C looks brownish. It's called brown charcoal. It's highly flammable and well suited for making black powder. But most charcoals for black powder purposes are charred at 400 °C.
Note: If you fill a plastic bottle with charcoal, tightly closed, the bottle will collapse after a few hours, since the coal binds the oxygen in the air in the bottle eagerly. Commercially run powder mills therefore store their charcoal in tin barrels containing not over 50 kilograms. Stored in a pile, this charcoal would self-ignite.
The influence of the kind of wood on the properties of black powder
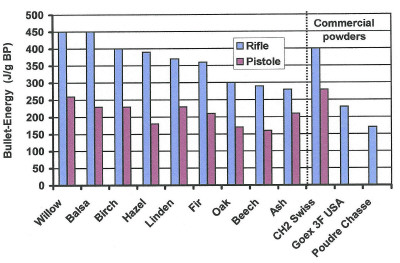
In Switzerland, the only remaining powder mill (poudrerie d'Aubonne) buys black alder fromYugoslavia as a raw material for charring. It's delivered as fagots already peeled. By the way, the peeling isn't done as a favor to the Swiss powder miller; - since the bark is marketed by the peelers as a laxative. In the middle ages, mostly willow and hazel wood were used. Grape wood is also mentioned. But, as I tested in the 1980s, any wood will provide a good black powder. As the graph below shows, most of them beat the commercially manufactured powders (last three columns).
 |
|||
| Fig. 3: The recipe of all these home-made powders was 100 parts saltpeter +17 parts sulfur + 16 parts charcoal charred at 400°C. The diagram shows the muzzle energy measured with a three band Enfield rifle Cal. 58 (blue columns) and a Mang Pistol Cal 38 (red columns). | |||
To compare the test powders with commercially available powders the last three tests were done with ..1) Swiss black powder, grade 2 from the poudrerie Aubonne. ..2) American Goex powder, grade 3F, ..3) French powder "poudre de chasse" (hunting powder).
For how the muzzle energy was measured see section entitled "Recipe"
Conclusions:
Not only to your surprise, dear visitor, also to my own surprise, it's that easy to stump the experts. I'm puzzled why on earth large powder mills are unable to make a decent black powder.
And note well: The secret of a good black powder is the charcoal alone !
Last revised: .Sept.12th, 2009