The Recipe for Black Powder
As is generally known to many people, black powder is a mixture of saltpeter, charcoal and sulfur. To disappoint all of you who think, in the following paper I'm going to give away an optimum recipe with the best possible performance, I state here once and for all: The mixture of the three ingredients is of little importance. It is the method of making the charcoal that counts, as I will prove with this chapter.
But back to the black powder recipe: You can change it over a wide range, and hardly notice any difference between performances. That explains why most of the historic recipes differ significantly. On the other hand, it is hard to find a historic recipe on how to char the coal, since that usually was a state secret.
By the way, even a mixture of saltpeter and charcoal without sulfur explodes remarkably well. However, a mixture of saltpeter and sulfur, without charcoal, doesn't burn at all. The latter fact puzzled the chemists throughout the centuries, because sulfur alone burns pretty well and saltpeter generally accelerates all sorts of fires.(Look this movie)
Note: Charcoal does not consist of pure carbon (C) as most authors purport. Such a pure charcoal would require a charring temperature of at least 1,500 °C! And a black powder mixed with it would probably burn like the head of a matchstick, at best.
As I'm explaining in my charcoal chapter, the formula of charcoal may be best described as C7H4O. With this and the following chemical equations we'll see why there won't be an explicit, best mixture of black powder:
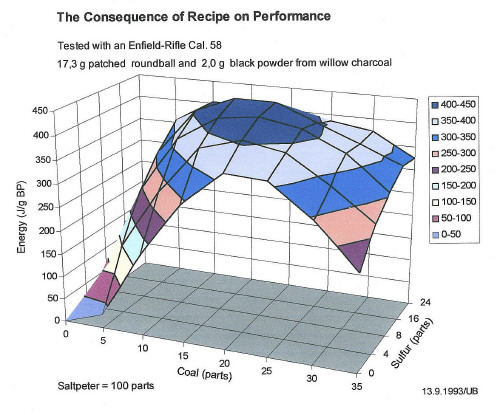
Depending on the portion of charcoal, its carbon produces alternatively carbon dioxide or carbon monoxide or a mix of both. To these you'll have to add the water vapor plus nitrogen, released from the saltpeter. So the sum of all the gases is nearly the same on all recipes. Indeed, the following three dimensional graph of the performance of different black powder recipes reveals a flat top over wide range of recipes.
A recipe without sulfur yields only little less energy (Joule/g). But its advantage lies in its little smoke produced. Its fog then consists of potassium carbonate (potash). Unfortunately its ignition temperature is 100 °C higher than usual. For cap lock arms that is no problem, but it is a nuisance to flint and match lock sportsmen.
Here is the chemical equation for a regular black powder containing sulfur:
| 1) | 4 KNO3 + C7H4O + 2 S | ------> | 2 K2S + 4 CO2 + 3CO + 2H2O + 2N2 | |||
| 2) | 6 KNO3 + C7H4O | ------> | 3 K2CO3 + CO2 + 6CO + 2H2O + 2N2 | ||||
|
KNO3
|
is saltpeter | |
| C7H4O | char coal | |
| S | sulfur |
|
| K2S | stands for potassiumsulfide (responsible for the white smoke of blackpowder) | |
| K2CO3 | pot ash | |
| CO | carbon monoxide | |
| CO2 | carbon dioxide | |
| N2 | Nitrogen |
Reasonable recipes for mixing blackpowder are;
A good standard black powder:..100 parts saltpeter + 18 parts coal + 16 parts sulfur
A powder without sulfur: ............100 parts saltpeter + 24 parts coal
As you may have noticed, I always give powder recipes on the basis of 100 parts saltpeter! Then, with the three ingredients, it leaves only two variables which can be compared or drawn on graphs easily.
For the creation of the following graph showing the performance of black powder, I tested dozens of recipes and fired some hundred shots.
The black powder samples were always mixed from saltpeter and sulfur supplied by the chemical industry. The charcoal I made from the wood of a willow tree, in fact from its young, debarked branches of approximately 3 to 4 cm diameter, charred in a tin can at 400 °C until nearly smokefree.
 |
||
| Fig. 1: The performance of different powder recipes |
||
The muzzle energy was determined by the measurement of the bullet velocity, one meter ahead of the muzzle of a three band Enfield rifle, cal. 58 (14.7 mm) and a patched round ball of 17.3 g lead. The velocity was measured by a commercially available chronograph. With each recipe there were three shots fired and from these the median was taken. The load was exactly 2.00 g black powder.
Then the muzzle energy was calculated as:
| Energy (Joule) = Mass of the ball (kg) • velocity2 (m/s)2 | |||
| 3) | |||
Historical black powder recipes
The first written recipe comes from Roger Bacon, a British monk and professor of philosophy at the university of Paris, in his "Opus Tertiary" (1264-1268),. There he wrote "As is widely known ..... " and then he gives the recipe. Note, he didn't claim the invention for himself and, therefore, he is definitely not the inventor. His recipe is:
Sed tamen 7 Partes Salpetrae, 5 Partes Coruli et 5 Partes Sulphuris et sic facies tonitrum et coruscationem, sic scias artificium.
Translated: Take 7 parts of saltpeter, 5 parts of hazelnut (-charcoal) and 5 parts sulfur and that makes thunder and flash if you know the art.
And later on in his text, he describes what bad boys were doing with it at night: - terrifiying their innocent neighborhood. They rolled paper to a tube, about the size of a thumb, filled it with powder, closed both ends with a piece of iron wire and then set it on fire". He didn't reveal exactly how the charge was ignited. By a fuse?
On the basis of 100 parts of saltpeter, Bacons recipe is: 100 p Saltpeter, 71 p coal, 71 p sulfur.
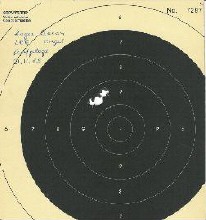
When tested, Bacons powder burned very slowly. I tested it on a handgonne (not a handgun). It didn't even propel the ball out of the barrel. All the pressure escaped through the touch hole. So I assumed, Bacon as an Englishman measured his parts by volume and not by weight as we continental Europeans are used to do. Even today, all English give their cooking recipes in cups and spoons and not by weight.
Did Bacon actually mean parts by volume (?), so his recipe would be by weight:
100 p Saltpeter + 27 p Coal + 45 p Sulfur
 |
||
| Fig 2: And here is what I shot with Bacons recipe by volume: 3 shots bench-rest, at 25 meters with a muzzle loader pistol of cal. 38. |
||
Here are some other local recipes, used historically :
| Saltpeter parts |
Charcoal parts |
Sulfur parts |
|
| Marcus Graecus (ca. 1250) | 100 | 33 | 12 |
| Canton of Zurich (1775) | 100 | 16.7 | 16.7 |
| Swiss Army (1849) | 100 | 17.3 | 16.0 |
| Modern Swiss blackpowder | 100 | 20.0 | 13.3 |
According to Fig. 1, the muzzle energies of the latter three recipes cover the top of the blue cap of the diagram and hence can't be improved. That proves, the optimum mixture of black powder has been found not later than mid 18th century. But also the recipe of Marcus Graecus (exact date uncertain) shows a good performance.
The role of Sulfur in black powder
Contemplating the diagram in Fig. 1, you might ask yourself, why on earth did our ancestors take the trouble to add sulfur at all? That idea first occured to the Swiss artillery in 1875, when "smokeless black powder" was tested. Firing salvos out of a fortification soon made the enemy invisible, due to the heavy smoke of the hefty loads of cannons. Since those tests were done on the eve of the "white-powder" era, the findings soon became obsolete.
A sulfur-free black powder yields only little smoke. When it is used in a rifle, you see only a faint fog. When fired by a percussion rifle, the ignition poses no problems. From by flint lock, you will get lots of misfires.
So I tested the ignition temperature of (homemade) black powders by increasing the temperature in a laboratory oven in increments of 10°C and found the purpose of sulfur in the black powder mixture:
Ignition Temperatures:
| S'peter | Char- coal |
Sulfur | Ignition-Temp. | ||
| 100 p | - | 13 p | - | 12 p | 300 °C |
| 100 p | - | 27 p | - | 0 p | 440 °C |
p = parts
So it appears the sole purpose of sulfur in a black powder mixture is the reduction of the ignition temperature! Note: A good priming powder has allways a high sulfur concentration.