Saltpeter
No doubt, charcoal is the most influential ingredient for a good black powder. But saltpeter is the key ingredient. Without the discovery of saltpeter, black powder wouldn't exist.
The first time, black powder is mentioned is in the book of Marcus Graecus (Mark the Greek) titled: "Liber Ignum ad Comburendum Hostes" (Book of Fire for Burning Enemies). There are known six copies of this book, all written in Latin. The oldest, in the library of the Vatican in Rome, is from ca.1300; the most recent is from 1481. It contains 34 recipes for inextinguishable fires, self-igniting fires and rocket propellants, as well as two black powder recipes. If printed today, the text would fill about six pages. A good translation is given by J.R. Partington's "A History of Greek Fire and Gunpowder" (1960), p 42 -90.
For you and me the most important recipe is #14, saltpeter, written in Latin.
Quote:
Nota quod sal petrosum est minera terrae et reperitur in scopulis contra lapidus. Haec terra dissolvatur in aqua bulliente, postea depurata et distla per filtrum et permittatur per diem et noctem integram decoqui, et invenies in fundo laminas salis conjelatas cristallinas.
"Note, saltpeter is a mineral of the earth and appears as brushes on stone. This earth is dissolved in boiling water and thereafter purified by a filter. Then boil down this solution by day and night till plates of crystals appear at the bottom."
This is my own, literal, best translation, since many other authors and also Partington's translation, are inaccurate. Most authors puzzle about the Latin word "scopulis". The word has different meanings. All the authors are at one disadvantage: They never saw natural saltpeter and hence don't know what Graecus was talking about! Look at the photo below and you will know what Marcus Graecus had in mind when he wrote "scropulis".
| Fig. 1: A Saltpeter brush (efflorescence, ca. 5 cm) on a cellar wall of an old mill. Outside the house there once was a dung pit of a former horse stable. There are many of these efflorences in this cellar. I harvested about 0.2 kg of saltpeter there. |
||||
The next dispute among many black powder historians is about the quality of early saltpeter. Many doubt the effectiveness of ancient fire arms since they believe the first saltpeter was very crude and the chemists too dumb to purify it. Some state it was calcium nitrate and not potassium nitrate. So I took some brushes of the saltpeter efflorescence shown here and made an analysis without prior purification.
Analysis of the saltpeter brush above:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fig. 2: This analysis proves, natural saltpeter brushes contain 95.1% KNO3 (saltpeter) The "insoluble" consists of sandy particles scratched off the wall. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In this case it would be possible to mix a suitable black powder just with these scratched off saltpeter brushes. No prior purification of the saltpeter is mandatory here, though of course it's advisable to do so. The scratched off sand soon would abrade the barrel.
Also, statements that natural saltpeter is calcium nitrate are ridiculous. Calcium nitrate is very hygroscopic and doesn't form crystals at all, though it seems chemically possible that wet, nitrate rich soil with a lack of potassium balances its nitrate-ions with calcium.
The Manufacturing of Saltpeter
When I discuss the formation of saltpeter, I'm meaning natural saltpeter only. After 1915, it became possible for the chemical engineers to make saltpeter in any amounts by the Haber-Bosch synthesis and the Ostwald-process, using technical chemistry . But that won't be the topic here.
Soon after the writing of the "Liber Ignum" and the discovery of black powder ca. 1300, (see section "history") we hear from Roger Bacon in his book "Opus Tertium" (1275, look History) that black powder was used for fire crackers at first. For this purpose, there certainly was enough saltpeter to be scratched off from cellar walls.
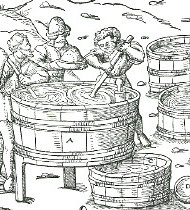
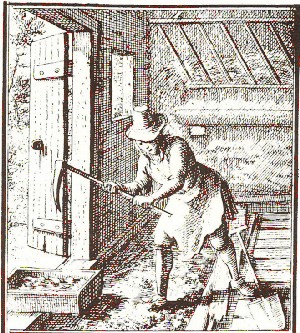
As soon as fire arms were invented, right after 1400, the powder mills might have become short of saltpeter. So the next source was cow stables. There saltpeter boilers (a distinctive profession) dug the soil from stables periodically and extracted the saltpeter according to Fig. 4.
 |
||||
| Fig 3: Saltpeter digger braking open the floor of a stable with a pick axe. This soil later will be processed according to Fig. 4 |
||||
Pictures 3 and 4 give a good impression of how the saltpeter was "mined" and later extracted from the soil dug in stables:
At first, the bottom of the extraction vat (Fig. 4) was covered with sand as a filter layer.
Then the vat was filled with saltpeter soil and leached with water. This lye was very turbid. This turbidity was flocculated by addition of some white of egg (A natural cationic polyelectrolyte a modern chemist would say today).
After the precipitation had settled, the cleared saltpeter solution was scooped into the reduction pan, a flat copper basin on a hearth. There, the solution boiled down to a concentrated saltpeter brine.
 |
||||
| Fig. 4: Georg Agricola's book:"Of mining and smelting" (1556) shows a saltpeter workshop. A) Pan for the reduction of the saltpeter brine B) Leeching of the saltpeter soil C) Plug D) Collector for the eluate E) Crystallization vat with copper sticks |
||||
Finally, the hot brine was poured into crystallization vats. The picture shows that these vats contain copper sticks, as crystallization nuclei. After crystallization, the saltpeter was scratched off the sticks and the rest of the brine went back to the reduction pan again. And so on.
Later, as the powder and saltpeter demand increased even more due to the continental-wide wars in Europe, stables didn't provide enough saltpeter. So, the next step was saltpeter plants.
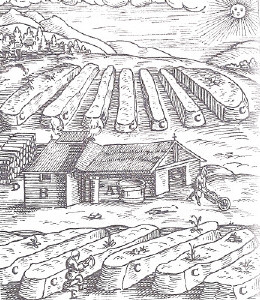 |
Fig. 5: Saltpeter plant: (16th century) Long rows of beds with porous walls(C), filled with a mixture of vegetable wastes, blood and dung as nitrogen source plus lime or ash to promote nitrification. Lime controls an optimum pH-value of 7.2 to 7.8. The porous walls of the beds allow an easy access of oxigen. In building (B) the contents of the ripe salpeter-beds were leached and saltpeter kristallised. (A) A vat, collecting rainwater from the roof. |
|||
| O. Guttmann:"Monumenta Pulveris Pyrii", London 1906. A collection of Reproductions of Ancient Pictures Concerning the History of Gunpowder |
||||
Only after 1890 was it understood, that bacteria played a roll in the oxidation of ammonium to nitrate. Never-the-less, from experience it was known that for an optimum yield, the saltpeter plants had to be inoculated with old saltpeter soil to prime the process and the manure had to be kept weakly alkaline by adding some lime or ash.
Today, this process isn't of interest anymore in regard to saltpeter production. But the process has been widely investigated in waste water treatment. Ammonium and especially ammonia are serious pollutants in creeks and rivers and, above all, toxic to most aquatic animals, especially fish. Hence it is the goal of any sewage treatment plant to oxidize the Ammonium of the raw waste water to harmless nitrate as far as possible..
The oxidation of ammonium to nitrate is done in two successive steps by two different kinds of bacteria
1st step: With the aid of nitrosomonas-germs ammonium is oxidized to nitrite
| 1) | NH3 |
+
|
1.5 O2 | -- Nitrosomonas --> | NO2 | + | H2O | + | 2 H+ |
| 2) | NO2 | + | 0.5 O2 | -- Spirobacter --> |
NO3
|
|---|
where:
| NH3 | = | ammonium |
| NO2 | = | nitrite |
| NO3 | = | nitrate |
Attention! The nitrification process works only under the following specific conditions:
- pH-value has to be within pH 6.8 and 7.8. Note the 2H+ in equation! 1).
When this acid isn't neutralized, the process will soon come to an end. - The temperature is more than 10 °C
- Nitrification bacts split once in 5 days at room temperature, so the process is very slow.
- It's significantly quicker at 35° (summertime).
- In wintertime there is no nitrification at all. Wait until the next summer!
Caption:
Fig 1: Photo by Ulrich Bretscher
Fig 3: Swiss National Library, Bern
Fig 4: Agricola, "Vom Berg- und Hüttenwesen" (1556)
Fig 5: O. Guttmann,"Monumenta Pulveris Pyrii", London 1906.
.........(A collection of Reproductions of Ancient Pictures Concerning the History of Gunpowder)
Last updated: Febr.15, 2010