Sulfur
Sulfur (S) is a chemical element with the following properties:
Atomic weight...............32 g/mol
Melting point...............114 °C
Boiling point................444 °C
Inflammation point..... 260 °C
Specific weight.............. 2.06 g/ml
Since antiquity and probably in prehistoric times, too, sulfur was mined in Sicily. Today a deposit consisting of a mix of sulfur, gypsum, lime and clay, containing typically about 25% sulfur, is still mined there. Sulfur is obtained by melting the sulfur from the excavated material in the absence of air. Later on, it is purified by distillation into a high quality product.
It is not true that this deposit has volcanic origins as many beleve. It is a former deposit on a deep sea floor. Alga and dead animals, whose proteins typically contain about 2% sulfur, settled on this ocean floor for thousends of years. Under anaerobic conditions (namely a redox potential of less than -300 mV), sulfur bacterias reduced this biological bound sulfur to sulfur-hydrogen and finally to the sulfur wich is minded today.
On the northern side of the Alps, in France and Germany, sulfur was made from pyrite (also called "fools gold") FeS2. When heated up to more than 450 °C this ore yields sulfur according to the following reaction:
| 2 FeS2 | ----> | 2 FeS | + | S2 |
Thanks to Georg Bäuerlein, alias "Agricola", we are well informed about how this was done. Agricola (1494-1555) was a physician from Chemnitz (Germany) who was an expert in mining and smelting. In 1556, his 12 volume treatise, "De re metallica", was published.. One year later, it appeared translated in German. This treatise covers the full contemporary knowledge of mining, smelting and illnesses of the miners and is illustrated with 250 woodprints. For several hundred years, it served as a standard for generations of miners and engineers. The following two illustrations about sulfur-smelting I took from a translation of the Latin reprint of 1928, issued again in1977. (A birthday gift from my wife Verena).
 |
 |
|||
| Fig. 1 | Fig. 2 | |||
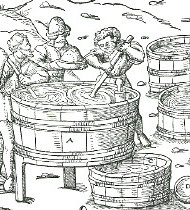
Fig. 1: This illustration shows a hearth covered with an iron plate. Agricola explains to us, that usually two pots were set atop this hearth (A) with spouts, filled with pyrite (FeS2) and closed by an iron cover. The pots’ spouts ended in another pot that served as cooler (B). Also this cooler was closed by an iron cover (C). The lid of this cover, as well as the covers of the pots with spouts, was sealed by clay. Also the holes in pot B that served as air cooler were sealed with clay to prevent the escape of sulfur fumes. In the cooler-pot, the sulfur vapor condensed to liquid sulfur and trickled into a vat beneath.
The liquid sulfur was then scooped from the vat into moulds to form bricks or rods. The wood print shows a miner casting sulfur bricks. In the lower right corner, we see a mould for casting sulfur rods. Obviously, the mould is fitted with a hinge for easy closing and opening.
Fig. 2: Another common method for sulfur smelting is shown in this illustration. Along a wall there is a bench. As a bench-top, we find massive square iron plates (D) with a hole in each. On this bench, centred with the holes in the plates, pots with many holes in bottoms (E) were placed . Then a fire was placed around these pots containing sulfur-ore or earth containing elementary sulfur. The molten sulfur then dropped through the holes in the bottom of the melting-pots and ran down into the collector, filled with water.
Today, most sulfur is consumed by the chemical industry, namely for the production of sulfuric acid. I still remember well the huge hills of sulfur chunks alongside a road leading from my home to the city of Zurich. They belonged to a company that produced sulfuric acid but quit in the 1970s. This sulfur was shipped in from Sicily.
Modern day sulfur production
Sulfur nowadays is mainly a waste product of the petroleum industry. Raw natural gas contains usually 0,1 - 0.5% hydrogen sulfide (H2S). Since hydrogen sulfide gas is extremely poisonous and an environmental pollutant, it is usually washed in sulfuric acid. This process yields sulfur dioxide and sulfur according to the following equation:
H2S + H2SO4 ----> S + SO2 + 2 H2O
The Swiss black powder contains sulfur from this process. It is delivered as sulfur flour.
Updated 18.8.2004